根据我国自然资源部发布的《2018年中国海洋经济统计公报》,2018年的全国海洋生产总值已达83415亿元,占国内生产总值的9.3%,航运业以及海洋相关产业已经成为当今经济发展的重要引擎。海洋环境复杂多变,高浓度的盐、溶解氧等因素共同作用造成海洋工程材料的严重腐蚀,带来巨大经济损失,埋下许多安全隐患。其中,微生物如细菌、真菌、古菌等在海洋装备灾难性事故中扮演的角色引起人们越来越多的关注。这种由于微生物生命活动或代谢产物所导致的腐蚀称为微生物腐蚀 (MIC)。
海洋中已经报道的微生物约有1500多种,具有腐蚀活性的微生物主要有硫酸盐还原菌 (SRB)、硝酸盐还原菌 (NRB)、铁氧化菌 (IOB)、铁还原菌 (IRB) 和产酸菌 (APB) 等[1],其主要通过参与Fe、S等的循环并改变金属表面的阴、阳极反应影响腐蚀过程[2-4]。自然环境下,金属的MIC往往伴随着生物膜的生成和发展,生物膜可以为微生物提供适合的生存环境,对杀菌剂起到屏障作用,且生物膜不同深度溶解氧、无机盐、有机营养成分等的浓度不同,为微生物之间的协同作用提供有利环境。MIC的控制在很大程度上是生物膜的控制[5,6]。SRB由于广泛存在于无氧环境,造成严重的腐蚀后果,被认为是最重要的腐蚀微生物。因此,MIC理论大多是以SRB为研究对象提出的,如经典的阴极去极化理论、浓差电池理论、代谢产物腐蚀理论等。而Jia等[6,7]在前人的基础上通过分析SRB、APB等微生物在金属腐蚀过程中的能量和电子传递过程,提出胞外电子传递理论 (EET-MIC)、代谢产物腐蚀理论 (M-MIC) 和生物降解腐蚀理论 (BD-MIC) 的划分,进一步完善了MIC理论。IRB作为海洋环境中一类重要的微生物,其对金属材料的腐蚀影响研究相对较少,有待进一步探索。
地球上的生命活动及进化与各类元素的循环息息相关,其中Fe作为地壳中含量第四位的元素,参与地球上大部分的生命代谢活动。现今的地球表面是一个固、液、气三相组成的高度异质环境,Fe主要以氧化态Fe(Ⅲ) 形式存在,但Fe(Ⅲ) 组成的矿物质或化合物溶解度极低,生物难以直接吸收。20世纪80年代以前,人们认为S的还原是微生物还原最早的形式,直到1987年分离并培养出第一株具有铁还原功能的细菌,人们开始意识到微生物还原Fe(Ⅲ) 可能是地球早期生命的呼吸方式之一,Vargas等[8]也通过实验证明了几种嗜热菌相比S更易还原Fe(Ⅲ)。近年来,随着不同种类的IRB被陆续从海水、海泥甚至深海热液区等水域环境中分离提取,其对金属材料的腐蚀作用也引起人们的关注[9]。虽然IRB对金属材料的腐蚀影响已取得一些成果,但对其作用结果及作用机制存在争议。因此,深入系统地探究IRB所致金属的腐蚀机制不仅可以进一步丰富MIC理论,还对海洋工程装备的防护具有理论指导意义。
本文从IRB种类的多样性、代谢过程的多样性、对金属材料的腐蚀机制研究现状、腐蚀研究方法等几个方面总结了海水环境中IRB所致金属材料腐蚀的研究进展。
1 海水环境中IRB种类的多样性
IRB并不是一个分类学上的概念,而是一类可以将Fe(Ⅲ) 还原为Fe(Ⅱ) 的微生物统称。Fe(Ⅲ) 的还原也称为异化铁还原,微生物通过氧化电子供体获得电子,以胞外Fe(Ⅲ) 作为末端电子受体,将Fe(Ⅲ) 还原为Fe(Ⅱ),同时获得能量[10]。IRB是一类严格厌氧或兼性厌氧的细菌或古菌,生存环境广泛,常见的海水环境中存在的IRB种类如表1所示。其中,深海热液区是重要的含铁氧化物的储存区,为异化IRB的生存繁殖提供了电子受体。但是,由于当前的技术限制,热液区的样品不易获取,并且外部条件改变后菌株不易存活,因此对深海热液区的IRB研究报道较少。目前在金属腐蚀领域研究最多的是Shewanella和Geobacter。
表1 海水环境已知的部分IRB种类
Table 1
| Genus | Original source | Characteristic description | |
|---|---|---|---|
| Bacterial | Desulfuromonas | Marine sediment | Gram-negative bacilli, obligate anaerobes, atrichic, c-cytochrome, symbiotic with Marinobacter [11] |
| Shewanella | Seawater, intertidal zone, marine sediment, marine organism, etc | Gram-negative bacilli, facultative anaerobic, single polar flagellum, optimal temperature 30 ℃, some of them symbiotic with Desulfovibrio, Brevibacillus and algae[12,13,14] | |
| Geobacter | Marine sediment | Gram-negative bacilli, obligate anaerobes, optimal temperature 22 ℃, symbiotic with Shewanella, or interspecies symbiosis[15] | |
| Rhodoferax | Bay sediment | Gram-negative bacilli, facultative anaerobic, single polar flagellum, optimal temperature 25 ℃, symbiotic with Azoarcus, Methanospirillum and Pseudomonas [16,17] | |
| Deferribacter | Arabian Sea, off-shore oil facility, deep hydrothermal vents (east pacific rise and the mid-atlantic ridge) | Obligate anaerobes or facultative anaerobic, multi-electron acceptor, symbiotic with Epsilonproteobacteria [18,19,20,21] | |
| Archaea | Hyperthermus | Deep hydrothermal zone | Obligate anaerobes, grow above 90 ℃, inactive under 80 ℃[22] |
| Geoglobus | Ashadze field | Only H2 as electron donor to reduce insoluble Fe(Ⅲ)[23] |
2 IRB代谢过程的多样性
微生物在代谢过程中需要不断地与外界进行物质交换,摄取能量,同时排出代谢产物,其本质是一系列的氧化还原反应,即存在电子供体和电子受体的电子传递过程。IRB从电子供体中获得电子,再将电子传递给Fe(Ⅲ) 使其还原,在此过程中获得并储存能量,有些IRB也可以还原其他有毒重金属和类金属,将还原过程作为自身的一种解毒机制[24]。
2.1 电子供体
IRB可利用H2、有机酸作为电子供体,尤其是短链有机酸,如乳酸盐、乙酸盐、甲酸盐,琥珀酸等;Geoglobus ahangari、Desulfuromonas palmitatis以及Geothrix fermentans可以利用一些长链脂肪酸如硬脂酸、棕榈酸等;还有少数几种可以利用葡萄糖和芳香族化合物作为电子供体,其中利用葡萄糖获得能量时大多通过发酵路径,产生乙酸。除此以外,极端环境分离的超嗜热菌如Ferroglobus placidus还能在一定条件下以Fe(Ⅱ) 为电子供体[25]。
2.2 电子受体
2.3 电子传递机制
IRB是通过向胞外转移电子的代谢方式实现电子传递过程。目前普遍认为,IRB通过以下几种机制传递电子 (见图1):(a) 直接接触机制:细菌以菌毛作为纳米导线或通过外膜上的活性蛋白,直接接触铁氧化物表面释放电子;(b) 电子穿梭机制:微生物分泌的氧化还原物质携带电子传递至胞外受体,再以氧化态返回胞内继续接受电子,如此往返于胞内外的介质;(c) 鳌合作用机制:微生物分泌出复杂的有机配位体与不溶性铁氧化物作用形成可溶性复合体,溶解性的Fe通过扩散作用到微生物表面,增大微生物与Fe(Ⅲ) 的接触效率,提高铁还原速率[31]。在实际环境中,这几种机制的贡献还不是很清楚。例如,Geobacter不能产生电子穿梭体及络合物,通常被认为主要是通过膜蛋白或产生鞭毛进行电子传递[32];而Shewanella能分泌电子穿梭体或螯合物,比如醌、有机配位基、黑色素等,直接接触可能就不作为其还原不溶性铁氧化物的主要途径[33,34]。
图1
图1
微生物作用于Fe(Ⅲ) 氧化物表面传递电子的3种方式[31]
Fig.1
Microbial strategies mediating electron transfer to insoluble Fe(Ⅲ) oxides: (a) direct contact with the oxide surface; (b) an endogenously or exogenously produced electron shuttle mediates electron transfer to solid-phase Fe(Ⅲ) oxides; (c) the production of ligands providing a soluble Fe(Ⅲ) form more readily available to the microorganism[31]
3 IRB对金属材料腐蚀的影响
金属在海水环境中的腐蚀过程大多是电化学过程,以钢铁的腐蚀为例,腐蚀过程中必定伴随着Fe的溶解,电极反应中的阳极反应为:
3.1 单一IRB作用下的腐蚀过程
3.1.1 腐蚀促进理论
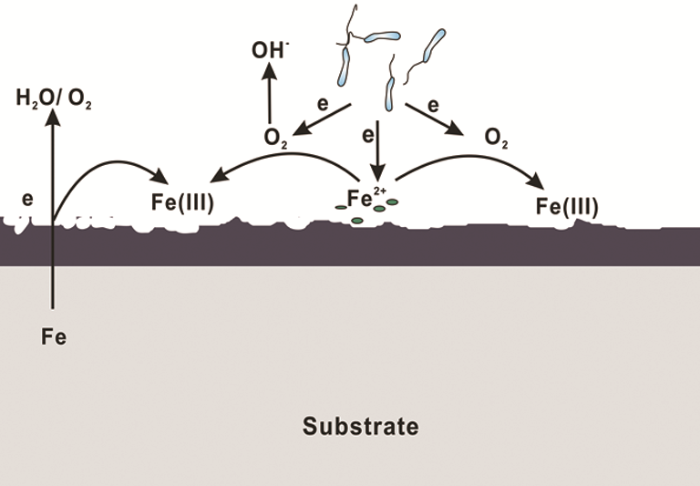
IRB引起金属材料腐蚀促进的MIC理论可总结为以下几方面:(1) 被广泛接受的腐蚀产物保护膜破坏理论。在一定条件下,钢铁表面形成的腐蚀产物膜对材料起到一定的保护作用,而IRB能够将保护性的Fe(III) 氧化物还原为可溶性的Fe(II),使得金属在腐蚀环境中呈现出新鲜的表面,加速金属腐蚀,其腐蚀机理如图2。上个世纪80年代,Obuekwe等[38]在微厌氧环境下研究了一株IRB (Pseudomonas sp.) 对碳钢腐蚀的影响,证明了IRB能够还原和移除由Fe(III) 化合物组成的保护性膜,促进阳极反应,进而加速碳钢腐蚀。之后,Little等[39]采用电化学噪声法研究了另外一株IRB (Shewanella purefaciens) 对碳钢腐蚀的影响,结果也表明IRB在碳钢表面通过将固态Fe(III) 的氧化物还原为可溶性的Fe(II),促进碳钢的腐蚀。(2) 局部腐蚀促进理论。IRB可形成不均匀生物膜,影响电化学过程,为局部腐蚀提供了条件,整体上表现为碳钢腐蚀被加速[36]。Chen等[40]采用丝束电极 (WBE) 的方法结合生物膜的形貌表征研究了一株IRB (Thalassospira. sp.) 在氧饱和海水中所致Q235碳钢局部腐蚀的动态过程,结果表明IRB的存在生成了不均匀的生物膜,且IRB在生物膜内的代谢导致了表面膜的局部破坏,产生了电化学活性位点,促进了Q235碳钢电荷转移过程。(3) 氢氧化腐蚀促进理论。随着电化学技术的发展,微区电化学技术已经在腐蚀领域得到越来越广泛的应用,因此有研究者研究了IRB和H2存在下碳钢的局部腐蚀[41,42],证明了IRB的氢氧化会使碳钢在短期内腐蚀加剧。
图2
图2
IRB引起的腐蚀促进机理图
Fig.2
Schematic illustration of the corrosion-enhanced process by IRB
3.1.2 腐蚀抑制理论
随着对IRB腐蚀作用研究的深入,越来越多的研究结果表明,IRB对金属材料起到腐蚀抑制作用,其原因可以总结为以下几点:(1) O2消耗腐蚀抑制理论。IRB的存在会直接或间接地消耗O2,抑制阴极反应从而抑制材料的腐蚀[43]。O2在金属腐蚀过程中起到重要作用,其氧化还原电位大于Fe(III)的,使得一些兼性IRB更倾向以O2作为电子受体以获得更多的能量;同时,被还原生成的Fe(II) 氧化进一步消耗O2,降低体系中的溶解氧,腐蚀机理模型见图3。(2) 保护性产物层腐蚀抑制理论。近期研究表明,一些IRB会诱导生成具有缓蚀性的保护层如蓝铁矿,从而抑制腐蚀。Cote等[44]认为Geobacter sulfurreducens以醋酸盐为碳源将Fe(III) 还原为Fe(II),而Fe (II) 与磷酸根结合生成磷酸亚铁 (Fe3(PO4)2·8H2O) 即蓝铁矿,沉积在碳钢表面,减少碳钢的腐蚀。Volkland等[45]也利用IRB在钢表面成功制备了蓝铁矿涂层,反应如下:
图3
图3
IRB引起的腐蚀抑制机理图
Fig.3
Schematic illustration of the corrosion-inhibited process by IRB
而Sun等[46]认为厌氧环境或梯度厌氧环境中IRB促进了Fe3O4的形成,延缓材料的腐蚀,反应式如下:
3.2 混合菌作用下的腐蚀过程
实际的海水环境是复杂多样的,MIC在多数情况下是不同微生物协同作用的结果。除了单一菌种,混合菌即两种及以上的菌种作用下金属的腐蚀行为也开始受到关注。其中,有研究者报道了IRB与IOB、铜绿假单胞菌等协同作用对金属腐蚀的影响。
IOB是常见的一类具有腐蚀活性的微生物,代谢过程中通过将Fe(II) 氧化成Fe(III) 获取能量,导致在金属表面产生氧浓差电池,引起局部腐蚀,腐蚀结果往往是在管道等金属表面形成锈瘤[49],锈瘤又为腐蚀性的厌氧微生物提供厌氧条件,从而造成金属严重的腐蚀。当IRB与IOB同时存在于体系中时,IOB以O2为电子受体,将Fe(II) 氧化成为Fe(III)。而IRB利用生成的Fe(III) 作为电子受体进行还原,但一些种类的IRB也倾向于以O2为电子受体,与IOB形成竞争。因此,二者的共同作用对金属腐蚀的影响值得探究。IRB与IOB的协同作用在淡水环境如自来水管道等方面研究较多。Wang等[50-52]的一系列研究证实,IRB与IOB通过在金属管道的内层和外层形成致密的腐蚀产物膜如α-FeOOH和Fe3O4等抑制管道的腐蚀。海水环境中其协同作用对腐蚀过程的影响研究较少。其中,Lee等[53]和Chen等[54]分别研究了不同种类的IRB和IOB混合菌作用对碳钢腐蚀的影响,结果均表明在有氧环境中IRB的加入会降低体系中O2含量,从而抑制阴极反应的进行,缓解IOB的腐蚀作用。
铜绿假单胞菌也是一种常见的微生物,广泛存在于各类环境中。Black等[55]在微好氧条件下,研究了低碳钢中铜绿假单胞菌和IRB共存的腐蚀效应,认为IRB去除保护性铁氧化物的能力导致铜绿假单胞菌进一步引起阳极极化导致腐蚀加速。
3.3 IRB影响腐蚀复杂性的原因剖析
IRB对海洋工程装备材料的腐蚀作用是不容忽视的,而现有的研究成果存在争议的主要原因可以概括为以下两点:
(1) IRB是一类具有铁还原能力的微生物,种类繁多,分布在不同的水域环境,因此,不同种类的IRB代谢过程中对电子受体和电子供体的选择不同。其中O2是一个影响IRB电子受体选择的重要因素,其氧化还原电位高于Fe(III),理论上有氧条件下兼性IRB趋向以O2作为电子受体,消耗体系中的O2,抑制腐蚀;而无氧条件下,IRB原则上以Fe(III) 等金属氧化物为电子受体,破坏腐蚀产物保护膜,可能会造成腐蚀加速。因此,不同种类的IRB在不同环境下对电子受体的选择不同,就会造成结果上的差异,很难进行系统对比。
(2) 生物膜的形成是一个非常复杂的生物/化学过程,对腐蚀过程的影响很难预测和控制,其组成受环境中微小扰动 (如温度、营养浓度和流量) 的影响,生物膜的状态会造成材料腐蚀的较大差异。现代的科学技术虽然已经在电化学检测、生物膜检测等方面得到了很大的改善,但生物膜内部的微环境如溶解氧、酸碱度、离子种类等还未能实现精确的跟踪检测。
4 IRB腐蚀影响的研究方法
4.1 腐蚀过程与产物测试
4.1.1 失重法
失重法测量腐蚀速率是一种简单可靠的方法。在IRB腐蚀影响的研究过程中,失重法被广泛应用。打磨后的样品在置于腐蚀体系中之前称重,浸泡一定的时间后,根据标准方法除去样品表面的生物膜及腐蚀产物后再称重,前后重量差值即为失重量。利用失重法可以得到一个直观、相对准确的腐蚀速率,但耗时较长且不易得到动态变化过程[6]。
4.1.2 电化学方法
相较于失重法,利用电化学手段研究IRB对金属表面腐蚀过程热力学和动力学过程的影响,可以得到快速准确的测试结果,是传统失重等方法不可比拟的。电化学阻抗谱 (EIS) 测试技术是研究金属腐蚀最常用的方法之一,该技术对被测体系扰动小,即使扰动信号长时间作用于电极,也不会给被测电极表面造成大的影响。极化曲线测量是腐蚀电化学研究的重要手段,线性极化 (LPR) 对研究电极在腐蚀电位附近外加电流进行微极化,通常选用相对开路电位±10 mV的扫描范围以最大限度接近体系的真实状态,通过计算电位对电流的斜率得到极化电阻 (Rp),可表征金属腐蚀速率;而通过Tafel极化得到的腐蚀电流 (icorr),不仅可以分析电极表面物质传输及腐蚀机理,还可以换算得到直观的腐蚀速率。
利用传统的电化学手段测试得到的是电极整体的电化学行为,不能提供某一微小区域的状态,微区电化学测试技术应运而生,弥补了普通电化学的不足。例如,局部电化学阻抗 (LEIS) 是通过向被测电极施加一个微扰电压,可以精确测量局部区域固/液界面的阻抗行为[56],从而分析局部腐蚀速率和生物膜下的差异。此外,扫描电化学显微镜 (SECM),扫描振动电极 (SVET)、扫描Kelvin探针 (SKP) 等微区电化学技术也得到相应的发展和应用。
4.1.3 腐蚀产物表征
金属表面的腐蚀产物形貌及成分分析对研究IRB影响下的腐蚀机制是不可或缺的。目前最常用的微观形貌表征手段有扫描电子显微镜 (SEM)、透射电子显微镜 (TEM),与其联用能谱 (EDS) 可进行元素分析。而腐蚀产物的物相分析常采用X射线衍射 (XRD)、X射线光电子能谱 (XPS)、Raman光谱分析等,多种方法相互验证,为确定产物成分提供可靠论证。
4.2 生物学研究方法
研究IRB对金属腐蚀的影响时,IRB的种类及其代谢特点、附着生物膜特征等信息必不可少,现代生物技术的发展为此提供了有力的工具。
4.2.1 IRB的分类
微生物的分类方法包括单菌株测序、宏基因高通量测序等。应用基因序列分析可鉴定生物之间的亲缘性,确定细菌分类及代谢特点,分析群落结构丰富度和多样性。其中,16SrRNA基因测序可以对特定环境下细菌和古菌的IRB种类和丰度进行有效的鉴定;而聚合酶链式反应技术 (PCR) 在短时间内大量扩增特定的DNA片段,用于序列分析、基因表达调控等。
4.2.2 生物膜表征
被微生物附着的样品经过超临界脱水后可以保留完整的微生物和胞外多聚物 (EPS) 形貌,在SEM下可以观察到其在金属表面的附着状态,但SEM无法给出细菌的存活状态和生物膜厚度。激光扫描共聚焦显微镜 (CLSM) 可以有效解决这一问题。观察前用不同的染料对含生物膜样品进行染色,生物膜中不同的成分与特定的染料结合,在CLSM下会激发出不同的荧光,确定生物膜中不同组分的分布情况。例如,用Live/Dead染料染色后的细菌,活细胞显示为绿色,而死细胞显示为红色,同时可以进行3D图像采集,既可以获得细菌在金属表面的存活状态,也可以获得直观的生物膜厚度。
4.2.3 基因敲除
分子生物学技术的发展使得基因敲除技术得到越来越广泛的应用,根据生物种类的不同,主要用到RecA系统同源重组法、CRISPR/Cas系统介导的基因敲除法和基于自杀载体的同源重组法等[57]。在IRB电子传递方式的研究过程中,通过敲除与电子传递相关的酶基因,可以进一步确定IRB的电子传递机制,Geobacter作为模式菌种已有所研究。例如,Summers等[58]利用基因敲除手段去除Geobacter sulfurreducens菌株中的色素蛋白Omcs (一种调节c型细胞色素的蛋白) 基因,再与Geobacter metallireducens构建共培养体系后证明了Geobacter sulfurreducens的导电纤毛参与种间直接电子传递的机制。Liu等[59]敲除Geobacter sulfurreducens菌株的Omcs基因后认为,纳米磁铁矿会促进Geobacter sulfurreducens和Geobacter metallireducens的直接电子传递过程。但是,由于IRB种类的复杂性和技术的局限,大部分IRB菌株还未见应用。在MIC研究领域,利用基因敲除技术去除与微生物腐蚀活性表达有关的基因,有助于明确微生物对金属腐蚀产生影响的直接因素,目前已应用在SRB、铜绿假单胞菌等所致金属腐蚀的研究中。例如,Huang等[60]通过基因敲除证实了铜绿假单胞菌中的phzM和phzS基因片段调控X80碳钢的EET-MIC。但是在IRB所致金属腐蚀的机制研究中,基因敲除的应用还比较少,具有较大的应用前景。
5 结语
综上所述,IRB作为一类重要的铁代谢菌,研究其对海水环境中金属的腐蚀影响意义重大。结合研究现状,针对存在的问题提出几点建议:第一,体系中参数的控制 (如溶解氧) 对研究IRB的腐蚀作用非常关键,尤其是有氧环境下应尽量减少溶解氧变化产生的影响,可以采用通氧或通空气的方式使体系内溶解氧保持浓度;第二,对生物膜内部微环境检测手段的提升和改善对研究IRB腐蚀影响机理具有重要意义;第三,IRB对O2的消耗容易造成贫氧环境,在实际海洋环境中能够为厌氧菌提供有利的生存环境,IRB与其他微生物混合作用所致腐蚀的机理需要进一步研究。
参考文献
The interaction of bacteria and metal surfaces
[J].
Microbiologically influenced corrosion of pipeline steels
[J].
管线钢的微生物腐蚀
[J].
Electron mediators accelerate the microbiologically influenced corrosion of 304 stainless steel by the Desulfovibriovulgaris biofilm
[J].In the microbiologically influenced corrosion (MIC) caused by sulfate reducing bacteria (SRB), iron oxidation happens outside sessile cells while the utilization of the electrons released by the oxidation process for sulfate reduction occurs in the SRB cytoplasm. Thus, cross-cell wall electron transfer is needed. It can only be achieved by electrogenic biofilms. This work hypothesized that the electron transfer is a bottleneck in MIC by SRB. To prove this, MIC tests were carried out using 304 stainless steel coupons covered with the Desulfovibrio vulgaris (ATCC 7757) biofilm in the ATCC 1249 medium. It was found that both riboflavin and flavin adenine dinucleotide (FAD), two common electron mediators that enhance electron transfer, accelerated pitting corrosion and weight loss on the coupons when 10ppm (w/w) of either of them was added to the culture medium in 7-day anaerobic lab tests. This finding has important implications in MIC forensics and biofilm synergy in MIC that causes billions of dollars of damages to the US industry each year.
Potentiodynamic polarization behaviour of AISI type 316 stainless steel in NaCl solution
[J].
Biofilms: Strategies for metal corrosion inhibition employing microorganisms
[J].Corrosion causes dramatic economic loss. Currently widely used corrosion control strategies have disadvantages of being expensive, subject to environmental restrictions, and sometimes inefficient. Studies show that microbial corrosion inhibition is actually a common phenomenon. The present review summarizes recent progress in this novel strategy: corrosion control using beneficial bacteria biofilms. The possible mechanisms may involve: (1) removal of corrosive agents (such as oxygen) by bacterial physiological activities (e.g., aerobic respiration), (2) growth inhibition of corrosion-causing bacteria by antimicrobials generated within biofilms [e.g., sulfate-reducing bacteria (SRB) corrosion inhibition by gramicidin S-producing Bacillus brevis biofilm], (3) generation of protective layer by biofilms (e.g., Bacillus licheniformis biofilm produces on aluminum surface a sticky protective layer of gamma-polyglutamate). Successful utilization of this novel strategy relies on advances in study at the interface of corrosion engineering and biofilm biology.
Microbiologically influenced corrosion and current mitigation strategies: A state of the art review
[J].
Electron transfer mediators accelerated the microbiologically influence corrosion against carbon steel by nitrate reducing Pseudomonas aeruginosa biofilm
[J].Electron transfer is a rate-limiting step in microbiologically influenced corrosion (MIC) caused by microbes that utilize extracellular electrons. Cross-cell wall electron transfer is necessary to transport the electrons released from extracellular iron oxidation into the cytoplasm of cells. Electron transfer mediators were found to accelerate the MIC caused by sulfate reducing bacteria. However, there is no publication in the literature showing the effect of electron transfer mediators on MIC caused by nitrate reducing bacteria (NRB). This work demonstrated that the corrosion of anaerobic Pseudomonas aeruginosa (PAO1) grown as a nitrate reducing bacterium biofilm on C1018 carbon steel was enhanced by two electron transfer mediators, riboflavin and flavin adenine dinucleotide (FAD) separately during a 7-day incubation period. The addition of either 10ppm (w/w) (26.6muM) riboflavin or 10ppm (12.7muM) FAD did not increase planktonic cell counts, but they increased the maximum pit depth on carbon steel coupons considerably from 17.5mum to 24.4mum and 25.0mum, respectively. Riboflavin and FAD also increased the specific weight loss of carbon steel from 2.06mg/cm(2) to 2.34mg/cm(2) and 2.61mg/cm(2), respectively. Linear polarization resistance, electrochemical impedance spectroscopy and potentiodynamic polarization curves all corroborated the pitting and weight loss data.
Microbiological evidence for Fe(III) reduction on early Earth
[J].It is generally considered that sulphur reduction was one of the earliest forms of microbial respiration, because the known microorganisms that are most closely related to the last common ancestor of modern life are primarily anaerobic, sulphur-reducing hyperthermophiles. However, geochemical evidence indicates that Fe(III) is more likely than sulphur to have been the first external electron acceptor of global significance in microbial metabolism. Here we show that Archaea and Bacteria that are most closely related to the last common ancestor can reduce Fe(III) to Fe(II) and conserve energy to support growth from this respiration. Surprisingly, even Thermotoga maritima, previously considered to have only a fermentative metabolism, could grow as a respiratory organism when Fe(III) was provided as an electron acceptor. These results provide microbiological evidence that Fe(III) reduction could have been an important process on early Earth and suggest that microorganisms might contribute to Fe(III) reduction in modern hot biospheres. Furthermore, our discovery that hyperthermophiles that had previously been thought to require sulphur for cultivation can instead be grown without the production of toxic and corrosive sulphide, should aid biochemical investigations of these poorly understood organisms.
Progress on influence of cathodic polarization on sulfate-reducing bacteria induced corrosion
[J].
阴极极化对硫酸盐还原菌腐蚀影响的研究进展
[J].介绍了硫酸盐还原菌 (SRB) 的生态和生理特征及在含SRB的环境中金属材料阴极保护的可靠性;重点综述了阴极极化对SRB腐蚀的影响,包括阴极极化对金属材料氢脆和力学性能、金属构筑物周围环境和微生物的影响;最后展望了微生物腐蚀研究的近期发展趋势。
Halotolerant bioanodes: The applied potential modulates the electrochemical characteristics, the biofilm structure and the ratio of the two dominant genera
[J].The development of economically-efficient microbial electrochemical technologies remains hindered by the low ionic conductivity of the culture media used as the electrolyte. To overcome this drawback, halotolerant bioanodes were designed with salt marsh sediment used as the inoculum in electrolytes containing NaCl at 30 or 45g/L (ionic conductivity 7.0 or 10.4S.m(-1)). The bioanodes were formed at four different potentials -0.4, -0.2, 0.0 and 0.2V/SCE to identify the effect on the electrochemical kinetic parameters, the biofilm structures and the composition of the microbial communities. The bioanodes formed at -0.4V/SCE were largely dominated by Marinobacter spp. Voltammetry showed that they provided higher currents than the other bioanodes in the range of low potentials, but the maximum currents were limited by the poor surface colonization. The bioanodes formed at -0.2, 0.0 and 0.2V/SCE showed similar ratios of Marinobacter and Desulfuromonas spp. and higher values of the maximum current density. The combined analysis of kinetic parameters, biofilm structure and biofilm composition showed that Marinobacter spp., which ensured a higher electron transfer rate, were promising species for the design of halotolerant bioanodes. The challenge is now to overcome its limited surface colonization in the absence of Desulfuromonas spp.
Characterization of microbial communities during anode biofilm reformation in a two-chambered microbial electrolysis cell (MEC)
[J].GeoChip (II) and single strand conformation polymorphism (SSCP) were used to characterize anode microbial communities of a microbial electrolysis cell (MEC). Biofilm communities, enriched in a two-chamber MEC (R1, 0.6 V applied) having a coulombic efficiency (CE) of 35+/-4% and a hydrogen yield (Y(H(2)))of 31+/-3%, were used as the inoculum for a new reactor (R2). After three months R2 achieved stable performance with CE=38+/-4% and (Y(H(2))). Few changes in the predominant populations were observed from R1 to R2. Unlike sludge inoculation process in R1 in the beginning, little further elimination was aroused by community competitions in anode biofilm reformation in R2. Functional genes detection of biofilm indicated that cytochrome genes enriched soon in new reactor R2, and four genera (Desulfovibrio, Rhodopseudomonas, Shewanella and Geobacter) were likely to contribute to exoelectrogenic activity. This work also implied that symbiosis of microbial communities (exoelectrogens and others) contribute to system performance and stability.
Community characteristics and ecological roles of bacterial biofilms associated with various algal settlements on coastal reefs
[J].
Enrichment and isolation of crude oil degrading bacteria from some mussels collected from the Persian Gulf
[J].
Stimulatory effect of magnetite on the syntrophic metabolism of Geobacter co-cultures: Influences of surface coating
[J].
Upgrading current method of anaerobic co-digestion of waste activated sludge for high-efficiency methanogenesis: Establishing direct interspecies electron transfer via ethanol-type fermentation
[J].
One-year monitoring of meta-cleavage dioxygenase gene expression and microbial community dynamics reveals the relevance of subfamily I.2.C extradiol dioxygenases in hypoxic, BTEX-contaminated groundwater
[J].Aromatic hydrocarbons including benzene, toluene, ethyl-benzene, and xylene (BTEX) are frequent contaminants of groundwater, the major drinking water resource. Bioremediation is the only sustainable process to clean up these environments. Microbial degradation of BTEX compounds occurs rapidly under aerobic conditions but, in subsurface environments, the availability of oxygen is commonly restricted. Even so, the microaerobic degradation of aromatic compounds is still poorly understood. Hence, the dynamics of a bacterial community and the expression of meta-cleavage dioxygenase genes, with particular emphasis on subfamily I.2.C extradiol dioxygenase genes, were assessed over a 13-month period in a hypoxic, aromatic hydrocarbon-contaminated shallow groundwater by using sequence-aided terminal-restriction fragment length polymorphism (T-RFLP) and single-nucleotide primer extension (SNuPE), respectively. The bacterial 16S rRNA fingerprinting revealed the predominance of members of Rhodoferax, Azoarcus, Pseudomonas, and unknown bacteria related to Rhodocyclaceae. It was observed that mRNA transcripts of subfamily I.2.C extradiol dioxygenase genes were detected constantly over the monitoring period, and the detected sequences clustered into six distinct clusters. In order to reveal changes in the expression of these clusters over the monitoring period a SNuPE assay was developed. This quasi fingerprinting of functional gene expression provided the opportunity to link the investigated function to specific microbial populations. The results obtained can improve our understanding of aromatic hydrocarbon degradation under oxygen limitation and may benefit bioremediation research by demonstrating the usefulness of SNuPE for the monitoring of microbial populations involved in degradation process.
16S rDNA-based bacterial diversity analysis of Arabian Sea sediments: A metagenomic approach
[J].
Succession of Deferribacteres and Epsilonproteobacteria through a nitrate-treated high-temperature oil production facility
[J].Members of Epsilonproteobacteria and Deferribacteres have been implied in nitrate-induced souring control in high-temperature oil production facilities. Here we report on their diversity and abundance in the injection and production part of a nitrate-treated, off-shore oil facility (Halfdan, Denmark) and aimed to assess their potential in souring control. Nitrate addition to deoxygenated seawater shifted the low-biomass seawater community dominated by Gammaproteobacteria closely affiliated with the genus Colwellia to a high-biomass community with significantly higher species richness. Epsilonproteobacteria accounted for less than 1% of the total bacterial community in the nitrate-amended injection water and were most likely outcompeted by putative nitrate-reducing, methylotrophic Gammaproteobacteria of the genus Methylophaga. Reservoir passage and recovery of the oil resulted in a significant change in the bacterial community. Members of the thermophilic Deferribacteres were the second major fraction of the bacterial community in the production water (~30% of the total bacterial community). They were not found in the injection water and were therefore assumed to be indigenous to the reservoir. Additional diversity analysis and targeted quantification of periplasmic nitrate reductase (napA) genes indicated that most resident Deferribacteres possessed the functional potential to contribute to nitrate reduction in the system. In sum, the dominance of nitrate-reducing Deferribacteres and the low relative abundance of Epsilonproteobacteria throughout the production facility suggested that the Deferribacteres play a major role in nitrate-induced souring control at high temperatures.
Evidence for the presence of thermophilic Fe(III)-reducing microorganisms in deep-sea hydrothermal vents at 13°N (East Pacific Rise)
[J].Microorganisms capable of dissimilatory Fe(III) reduction in the temperature range of 52-90 degrees C were enriched from outer and inner parts of chimney-like structures, tubes of the polychaetous annelid Alvinella sp., and hydrothermal fluids collected at 13 degrees N hydrothermal vent sites on the East Pacific Rise at a depth of 2650 m. Numbers of culturable Fe(III)-reducing thermophiles estimated by the serial dilution technique varied from 10 to 10(7) cells per cm(3) of sample. Phylogenetic analysis of bacterial and archaeal PCR-amplified 16S rDNA genes obtained from Fe(III)-reducing enrichments and separated by denaturing gradient gel electrophoresis revealed sequences related to Deferibacter, Thermotogales (Bacteria) and Thermococcus (Archaea) for which the capacity for Fe(III) reduction had been reported. This was confirmed by isolating a hyperthermophilic iron reducer that belongs to the genus Thermococcus. Other bacterial thermophiles found in the enrichments were related to so far uncultured members of the Clostridiaceae, and epsilon-subdivision of the Proteobacteria.
Deferribacter autotrophicus sp. nov., an iron (III)-reducing bacterium from a deep-sea hydrothermal vent
[J].A thermophilic, anaerobic, chemolithoautotrophic bacterium (designated strain SL50(T)) was isolated from a hydrothermal sample collected at the Mid-Atlantic Ridge from the deepest of the known World ocean hydrothermal fields, Ashadze field (1 degrees 58' 21'' N 4 degrees 51' 47'' W) at a depth of 4100 m. Cells of strain SL50(T) were motile, straight to bent rods with one polar flagellum, 0.5-0.6 mum in width and 3.0-3.5 mum in length. The temperature range for growth was 25-75 degrees C, with an optimum at 60 degrees C. The pH range for growth was 5.0-7.5, with an optimum at pH 6.5. Growth of strain SL50(T) was observed at NaCl concentrations ranging from 1.0 to 6.0 % (w/v) with an optimum at 2.5 % (w/v). The generation time under optimal growth conditions for strain SL50(T) was 60 min. Strain SL50(T) used molecular hydrogen, acetate, lactate, succinate, pyruvate and complex proteinaceous compounds as electron donors, and Fe(III), Mn(IV), nitrate or elemental sulfur as electron acceptors. The G+C content of the DNA of strain SL50(T) was 28.7 mol%. 16S rRNA gene sequence analysis revealed that the closest relative of strain SL50(T) was Deferribacter abyssi JR(T) (95.5 % similarity). On the basis of its physiological properties and phylogenetic analyses, the isolate is considered to represent a novel species, for which the name Deferribacter autotrophicus sp. nov. is proposed. The type strain is SL50(T) (=DSM 21529(T)=VKPM B-10097(T)). Deferribacter autotrophicus sp. nov. is the first described deep-sea bacterium capable of chemolithoautotrophic growth using molecular hydrogen as an electron donor and ferric iron as electron acceptor and CO(2) as the carbon source.
Magnetite formation from ferrihydrite by hyperthermophilic archaea from Endeavour Segment, Juan de Fuca Ridge hydrothermal vent chimneys
[J].
Geoglobus acetivorans sp. nov., an iron (III)-reducing archaeon from a deep-sea hydrothermal vent
[J].A hyperthermophilic, anaerobic, dissimilatory Fe(III)-reducing, facultatively chemolithoautotrophic archaeon (strain SBH6(T)) was isolated from a hydrothermal sample collected from the deepest of the known World Ocean hydrothermal fields, Ashadze field (1 degrees 58' 21'' N 4 degrees 51' 47'' W) on the Mid-Atlantic Ridge, at a depth of 4100 m. The strain was enriched using acetate as the electron donor and Fe(III) oxide as the electron acceptor. Cells of strain SBH6(T) were irregular cocci, 0.3-0.5 mum in diameter. The temperature range for growth was 50-85 degrees C, with an optimum at 81 degrees C. The pH range for growth was 5.0-7.5, with an optimum at pH 6.8. Growth of SBH6(T) was observed at NaCl concentrations ranging from 1 to 6 % (w/v) with an optimum at 2.5 % (w/v). The isolate utilized acetate, formate, pyruvate, fumarate, malate, propionate, butyrate, succinate, glycerol, stearate, palmitate, peptone and yeast extract as electron donors for Fe(III) reduction. It was also capable of growth with H(2) as the sole electron donor, CO(2) as a carbon source and Fe(III) as an electron acceptor without the need for organic substances. Fe(III) [in the form of poorly crystalline Fe(III) oxide or Fe(III) citrate] was the only electron acceptor that supported growth. 16S rRNA gene sequence analysis revealed that the closest relative of the isolated organism was Geoglobus ahangari 234(T) (97.0 %). On the basis of its physiological properties and phylogenetic analyses, the isolate is considered to represent a novel species, for which the name Geoglobus acetivorans sp. nov. is proposed. The type strain is SBH6(T) (=DSM 21716(T) =VKM B-2522(T)).
DissImilatory Fe(III) reduction by microorganisms
[J].
Fe(III) 的微生物异化还原
[J].
Mechanisms involved in Fe(III) respiration by the hyperthermophilic archaeon Ferroglobus placidus
[J].
Role of iron-reducing bacteria in corrosion and protection of carbon steel
[J].
Research on corrosion behavior of Q235 steel in marine iron-oxidizing bacteria
[J].
Q235钢在海洋铁细菌作用下的腐蚀行为研究
[J].
Impact of Fe(II) oxidation in the presence of iron-reducing bacteria on subsequent Fe(III) bio-reduction
[J].
Fe(III) reduction during pyruvate fermentation by Desulfotomaculum reducens strain MI-1
[J].
Ferric iron reduction by fermentative strain BS2 isolated from an iron-rich anoxic environment (Lake Pavin, France)
[J].
Microorganisms pumping iron: Anaerobic microbial iron oxidation and reduction
[J].Iron (Fe) has long been a recognized physiological requirement for life, yet for many microorganisms that persist in water, soils and sediments, its role extends well beyond that of a nutritional necessity. Fe(II) can function as an electron source for iron-oxidizing microorganisms under both oxic and anoxic conditions and Fe(III) can function as a terminal electron acceptor under anoxic conditions for iron-reducing microorganisms. Given that iron is the fourth most abundant element in the Earth's crust, iron redox reactions have the potential to support substantial microbial populations in soil and sedimentary environments. As such, biological iron apportionment has been described as one of the most ancient forms of microbial metabolism on Earth, and as a conceivable extraterrestrial metabolism on other iron-mineral-rich planets such as Mars. Furthermore, the metabolic versatility of the microorganisms involved in these reactions has resulted in the development of biotechnological applications to remediate contaminated environments and harvest energy.
Lack of production of electron-shuttling compounds or solubilization of Fe(III) during reduction of insoluble Fe(III) oxide by Geobacter metallireducens
[J].Studies with the dissimilatory Fe(III)-reducing microorganism Geobacter metallireducens demonstrated that the common technique of separating Fe(III)-reducing microorganisms and Fe(III) oxides with semipermeable membranes in order to determine whether the Fe(III) reducers release electron-shuttling compounds and/or Fe(III) chelators is invalid. This raised doubts about the mechanisms for Fe(III) oxide reduction by this organism. However, several experimental approaches indicated that G. metallireducens does not release electron-shuttling compounds and does not significantly solubilize Fe(III) during Fe(III) oxide reduction. These results suggest that G. metallireducens directly reduces insoluble Fe(III) oxide.
Outer membrane-associated serine protease involved in adhesion of Shewanella oneidensis to Fe(Ⅲ) oxides
[J].
Shewanella secretes flavins that mediate extracellular electron transfer
[J].Bacteria able to transfer electrons to metals are key agents in biogeochemical metal cycling, subsurface bioremediation, and corrosion processes. More recently, these bacteria have gained attention as the transfer of electrons from the cell surface to conductive materials can be used in multiple applications. In this work, we adapted electrochemical techniques to probe intact biofilms of Shewanella oneidensis MR-1 and Shewanella sp. MR-4 grown by using a poised electrode as an electron acceptor. This approach detected redox-active molecules within biofilms, which were involved in electron transfer to the electrode. A combination of methods identified a mixture of riboflavin and riboflavin-5'-phosphate in supernatants from biofilm reactors, with riboflavin representing the dominant component during sustained incubations (>72 h). Removal of riboflavin from biofilms reduced the rate of electron transfer to electrodes by >70%, consistent with a role as a soluble redox shuttle carrying electrons from the cell surface to external acceptors. Differential pulse voltammetry and cyclic voltammetry revealed a layer of flavins adsorbed to electrodes, even after soluble components were removed, especially in older biofilms. Riboflavin adsorbed quickly to other surfaces of geochemical interest, such as Fe(III) and Mn(IV) oxy(hydr)oxides. This in situ demonstration of flavin production, and sequestration at surfaces, requires the paradigm of soluble redox shuttles in geochemistry to be adjusted to include binding and modification of surfaces. Moreover, the known ability of isoalloxazine rings to act as metal chelators, along with their electron shuttling capacity, suggests that extracellular respiration of minerals by Shewanella is more complex than originally conceived.
Toward a better understanding of microbiologically influenced corrosion caused by sulfate reducing bacteria
[J].
A perspective on corrosion inhibition by biofilms
[J].
Understanding microbial inhibition of corrosion. A comprehensive overview
[J].
Corrosion of mild steel in cultures of ferric iron reducing bacterium isolated from crude oil i. polarization characteristics
[J].
The role of biomineralization in microbiologically influenced corrosion
[J].Synthetic iron oxides (goethite, alpha-FeO.OH; hematite, Fe2O3; and ferrihydrite, Fe(OH)3) were used as model compounds to simulate the mineralogy of surface films on carbon steel. Dissolution of these oxides exposed to pure cultures of the metal-reducing bacterium, Shewanella putrefaciens, was followed by direct atomic absorption spectroscopy measurement of ferrous iron coupled with microscopic analyses using confocal laser scanning and environmental scanning electron microscopies. During an 8-day exposure the organism colonized mineral surfaces and reduced solid ferric oxides to soluble ferrous ions. Elemental composition, as monitored by energy dispersive x-ray spectroscopy, indicated mineral replacement reactions with both ferrihydrite and goethite as iron reduction occurred. When carbon steel electrodes were exposed to S. putrefaciens, microbiologically influenced corrosion was demonstrated electrochemically and microscopically.
Corrosion behavior of Q235 carbon steel in air-saturated seawater containing Thalassospira sp
[J].
Influence of hydrogen-oxidizing bacteria on the corrosion of low carbon steel: Local electrochemical investigations
[J].Low carbon steel has been considered a suitable material for component of the multi-barrier system employed on the geological disposal of high-level radioactive waste (HLW). A non negligible amount of dihydrogen (H2) is expected to be produced over the years within the geological repository due to the anoxic corrosion of metallic materials and also to the water radiolysis. The influence of the activity of hydrogen-oxidizing bacteria (HOB) and iron-reducing bacteria (IRB) on carbon steel corrosion is considered in this study because of the high availability of energetic nutriments (H2, iron oxides and hydroxides) produced in anoxic disposal conditions. Local electrochemical techniques were used for investigating the activity of IRB as a promoter of local corrosion in the presence of H2 as electron donor. A local consumption of H2 by the bacteria has been evidenced and impedance measurements indicate the formation of a thick layer of corrosion products.
Metallic corrosion processes reactivation sustained by iron-reducing bacteria: Implication on long-term stability of protective layers
[J].
Microbial iron respiration can protect steel from corrosion
[J].
Geobacter sulfurreducens: An iron reducing bacterium that can protect carbon steel against corrosion?
[J].
Repair of damaged vivianite coatings on mild steel using bacteria
[J].
Formation and release behavior of iron corrosion products under the influence of bacterial communities in a simulated water distribution system
[J].
Effects of disinfectant and biofilm on the corrosion of cast iron pipes in a reclaimed water distribution system
[J].The effects of disinfection and biofilm on the corrosion of cast iron pipe in a model reclaimed water distribution system were studied using annular reactors (ARs). The corrosion scales formed under different conditions were characterized by X-ray diffraction (XRD), energy dispersive spectroscopy (EDS), and scanning electron microscopy (SEM), while the bacterial characteristics of biofilm on the surface were determined using several molecular methods. The corrosion scales from the ARs with chlorine included predominantly alpha-FeOOH and Fe2O3, while CaPO3(OH)center dot 2H(2)O and alpha-FeOOH were the predominant phases after chloramines replaced chlorine. Studies of the consumption of chlorine and iron release indicated that the formation of dense oxide layers and biofilm inhibited iron corrosion, causing stable lower chlorine decay. It was verified that iron-oxidizing bacteria (IOB) such as Sediminibacterium sp., and iron-reducing bacteria (IRB) such as Shewanella sp., synergistically interacted with the corrosion product to prevent further corrosion. For the ARs without disinfection, alpha-FeOOH was the predominant phase at the primary stage, while CaCO3 and alpha-FeOOH were predominant with increasing time. The mixed corrosion-inducing bacteria, including the IRB Shewanella sp., the IOB Sediminibacterium sp., and the sulfur-oxidizing bacteria (SOB) Limnobacter thioxidans strain, promoted iron corrosion by synergistic interactions in the primary period, while anaerobic IRB became the predominant corrosion bacteria, preventing further corrosion via the formation of protective layers. (C) 2011 Elsevier Ltd.
Microbial reduction of Fe(III) in hematite nanoparticles by Geobacter sulfurreducens
[J].The rates of microbial Fe(III) reduction of three sizes of hematite nanoparticles by Geobacter sulfurreducens were measured under two H2 partial pressures (0.01 and 1 atm) and three pH (7.0, 7.5, and 8.0) conditions. Hematite particles with mean primary particle sizes of 10, 30, and 50 nm were synthesized by a novel aerosol method that allows tight control of the particle size distribution. The mass-normalized reduction rates of the 10 and 30 nm particles were comparable to each other and higher than the rate for the 50 nm particles. However, the surface area-normalized rate was highest for the 30 nm particles. Consistent with a previously published model, the reduction rates are likely to be proportional to the bacteria-hematite contact area and not to the total hematite surface area. Surface area-normalized iron reduction rates were higher than those reported in previous studies, which may be due to the sequestration of Fe(II) through formation of vivianite. Similar initial reduction rates were observed under all pH and H2 conditions studied.
The corrosion behavior and mechanism of carbon steel induced by extracellular polymeric substances of iron-oxidizing bacteria
[J].
Effects of microbial redox cycling of iron on cast iron pipe corrosion in drinking water distribution systems
[J].Bacterial characteristics in corrosion products and their effect on the formation of dense corrosion scales on cast iron coupons were studied in drinking water, with sterile water acting as a reference. The corrosion process and corrosion scales were characterized by electrochemical and physico-chemical measurements. The results indicated that the corrosion was more rapidly inhibited and iron release was lower due to formation of more dense protective corrosion scales in drinking water than in sterile water. The microbial community and denitrifying functional genes were analyzed by pyrosequencing and quantitative polymerase chain reactions (qPCR), respectively. Principal component analysis (PCA) showed that the bacteria in corrosion products played an important role in the corrosion process in drinking water. Nitrate-reducing bacteria (NRB) Acidovorax and Hydrogenophaga enhanced iron corrosion before 6 days. After 20 days, the dominant bacteria became NRB Dechloromonas (40.08%) with the protective corrosion layer formation. The Dechloromonas exhibited the stronger corrosion inhibition by inducing the redox cycling of iron, to enhance the precipitation of iron oxides and formation of Fe3O4. Subsequently, other minor bacteria appeared in the corrosion scales, including iron-respiring bacteria and Rhizobium which captured iron by the produced siderophores, having a weaker corrosion-inhibition effect. Therefore, the microbially-driven redox cycling of iron with associated microbial capture of iron caused more compact corrosion scales formation and lower iron release. (C) 2014 Elsevier Ltd.
Characteristics of corrosion sales and biofilm in aged pipe distribution systems with switching water source
[J].
Characterization of biofilm bacterial communities and cast iron corrosion in bench-scale reactors with chloraminated drinking water
[J].
Iron cycling at corroding carbon steel surfaces
[J].Surfaces of carbon steel (CS) exposed to mixed cultures of iron-oxidizing bacteria (FeOB) and dissimilatory iron-reducing bacteria (FeRB) in seawater media under aerobic conditions were rougher than surfaces of CS exposed to pure cultures of either type of microorganism. The roughened surface, demonstrated by profilometry, is an indication of loss of metal from the surface. In the presence of CS, aerobically grown FeOB produced tight, twisted helical stalks encrusted with iron oxides. When CS was exposed anaerobically in the presence of FeRB, some surface oxides were removed. However, when the same FeOB and FeRB were grown together in an aerobic medium, FeOB stalks were less encrusted with iron oxides and appeared less tightly coiled. These observations suggest that iron oxides on the stalks were reduced and solubilized by the FeRB. Roughened surfaces of CS and denuded stalks were replicated with culture combinations of different species of FeOB and FeRB under three experimental conditions. Measurements of electrochemical polarization resistance established different rates of corrosion of CS in aerobic and anaerobic media, but could not differentiate rate differences between sterile controls and inoculated exposures for a given bulk concentration of dissolved oxygen. Similarly, total iron in the electrolyte could not be used to differentiate treatments. The experiments demonstrate the potential for iron cycling (oxidation and reduction) on corroding CS in aerobic seawater media.
Corrosion of Q235 carbon steel in seawater containing mariprofundus ferrooxydans and Thalassospira sp.
[J].Iron-oxidizing bacteria (IOB) and iron-reducing bacteria (IRB) can easily adhere onto carbon steel surface to form biofilm and affect corrosion processes. However, the mechanism of mixed consortium induced carbon steel corrosion is relatively underexplored. In this paper, the adsorptions of IOB (Mariprofundus ferrooxydans, M. f.), IRB (Thalassospira sp., T. sp.) and mixed consortium (M. f. and T. sp.) on surface of Q235 carbon steel and their effects on corrosion in seawater were investigated through surface analysis techniques and electrochemical methods. Results showed that local adhesion is a typical characteristic for biofilm on surface of Q235 carbon steel in M. f. and mixed consortium media, which induces localized corrosion of Q235 carbon steel. Corrosion rates of Q235 carbon steel in different culture media decrease in the order: r M.f. > r mixed consortium > r T. sp. > r sterile. The evolution of corrosion rate along with time decreases in M. f. medium, and increases then keeps table in both T. sp. and mixed consortium media. Corrosion mechanism of Q235 carbon steel in mixed consortium medium is discussed through analysis of surface morphology and composition, environmental parameter, and electrochemical behavior.
Evaluation of AISI Type 304 stainless steel as a suitable surface material for evaluating the efficacy of peracetic acid-based disinfectants against Clostridium difficile spores
[J].
Galvanic corrosion between zinc and carbon steel investigated by local electrochemical impedance spectroscopy
[J].
Advances in gene knockout techniques in microbiology
[J].
基因敲除技术在微生物中的应用
[J].
Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria
[J].Microbial consortia that cooperatively exchange electrons play a key role in the anaerobic processing of organic matter. Interspecies hydrogen transfer is a well-documented strategy for electron exchange in dispersed laboratory cultures, but cooperative partners in natural environments often form multispecies aggregates. We found that laboratory evolution of a coculture of Geobacter metallireducens and Geobacter sulfurreducens metabolizing ethanol favored the formation of aggregates that were electrically conductive. Sequencing aggregate DNA revealed selection for a mutation that enhances the production of a c-type cytochrome involved in extracellular electron transfer and accelerates the formation of aggregates. Aggregate formation was also much faster in mutants that were deficient in interspecies hydrogen transfer, further suggesting direct interspecies electron transfer.
Magnetite compensates for the lack of a pilin-associated c-type cytochrome in extracellular electron exchange
[J].Nanoscale magnetite can facilitate microbial extracellular electron transfer that plays an important role in biogeochemical cycles, bioremediation and several bioenergy strategies, but the mechanisms for the stimulation of extracellular electron transfer are poorly understood. Further investigation revealed that magnetite attached to the electrically conductive pili of Geobacter species in a manner reminiscent of the association of the multi-heme c-type cytochrome OmcS with the pili of Geobacter sulfurreducens. Magnetite conferred extracellular electron capabilities on an OmcS-deficient strain unable to participate in interspecies electron transfer or Fe(III) oxide reduction. In the presence of magnetite wild-type cells repressed expression of the OmcS gene, suggesting that cells might need to produce less OmcS when magnetite was available. The finding that magnetite can compensate for the lack of the electron transfer functions of a multi-heme c-type cytochrome has implications not only for the function of modern microbes, but also for the early evolution of microbial electron transport mechanisms.
Pyocyanin-modifying genes phzM and phzS regulated the extracellular electron transfer in microbiologically-influenced corrosion of X80 carbon steel by Pseudomonas aeruginosa
[J].